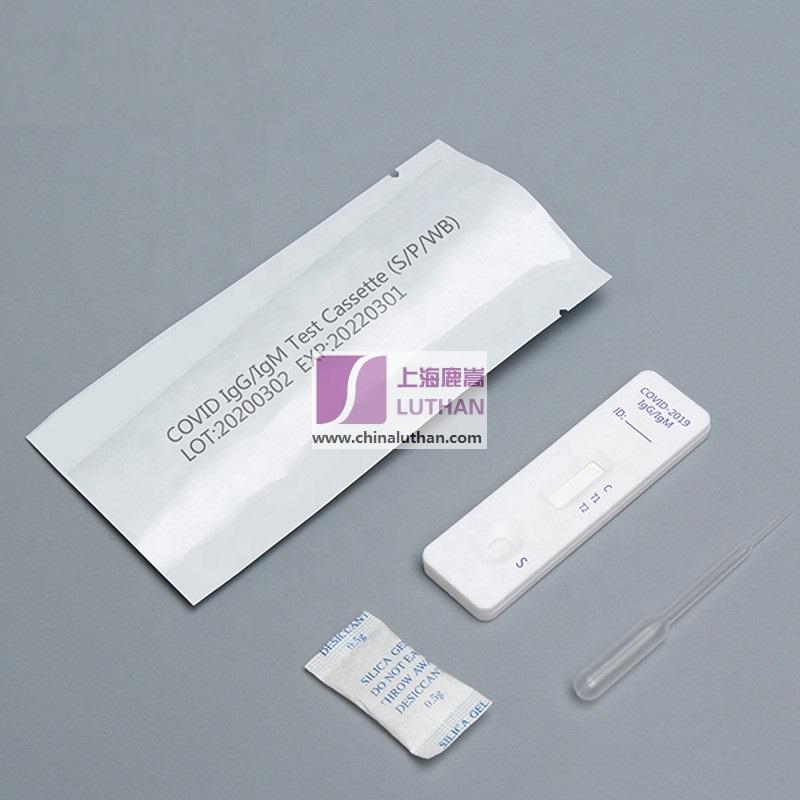
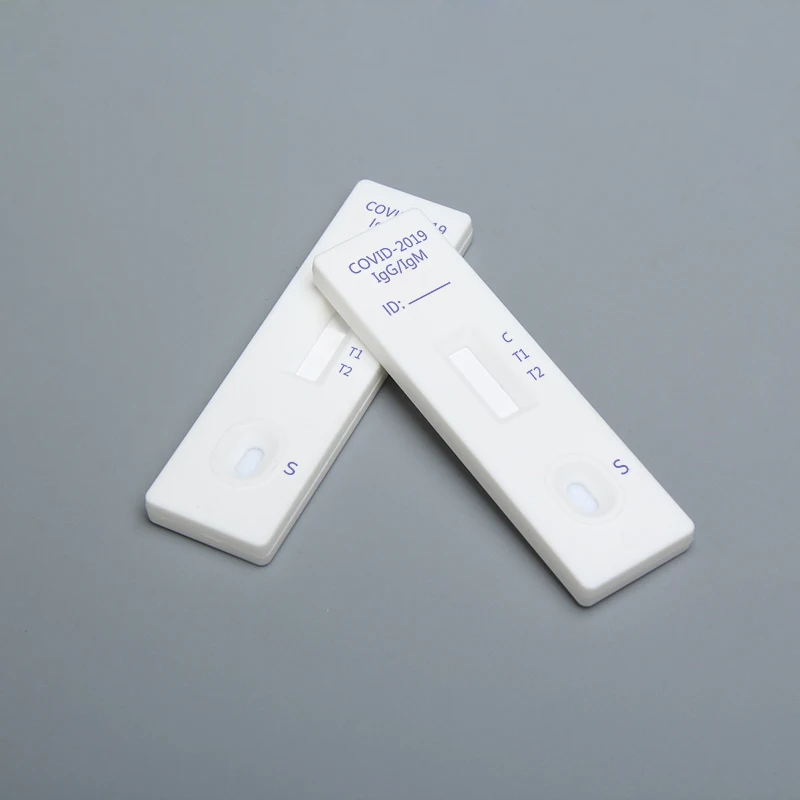
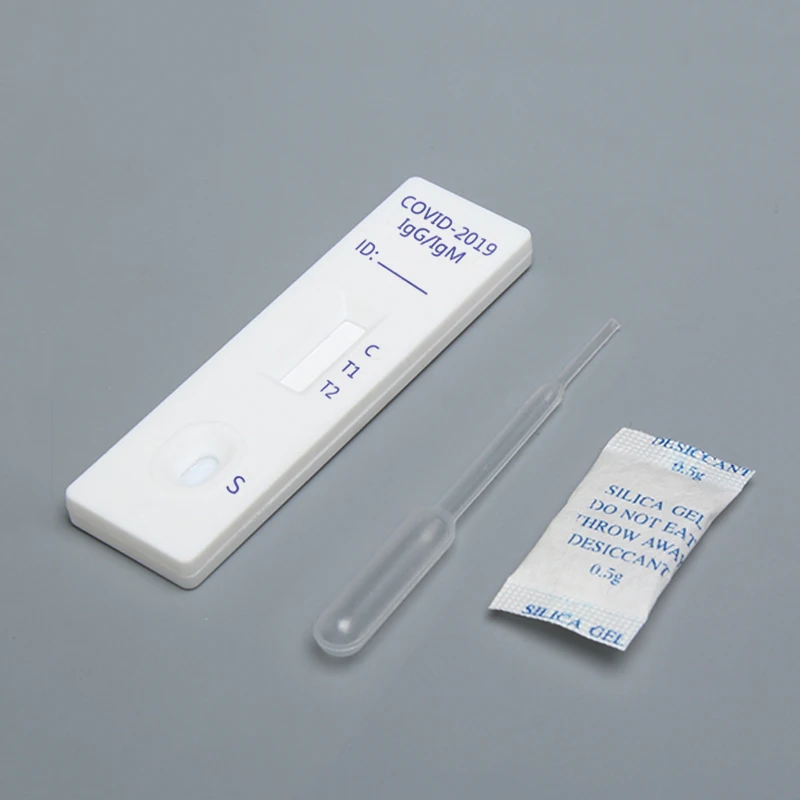
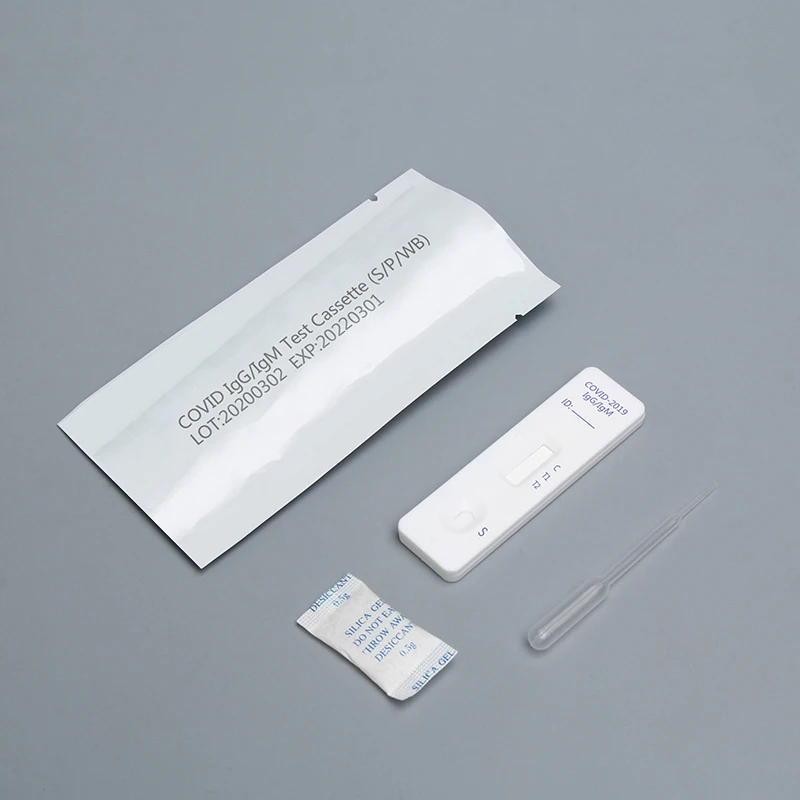
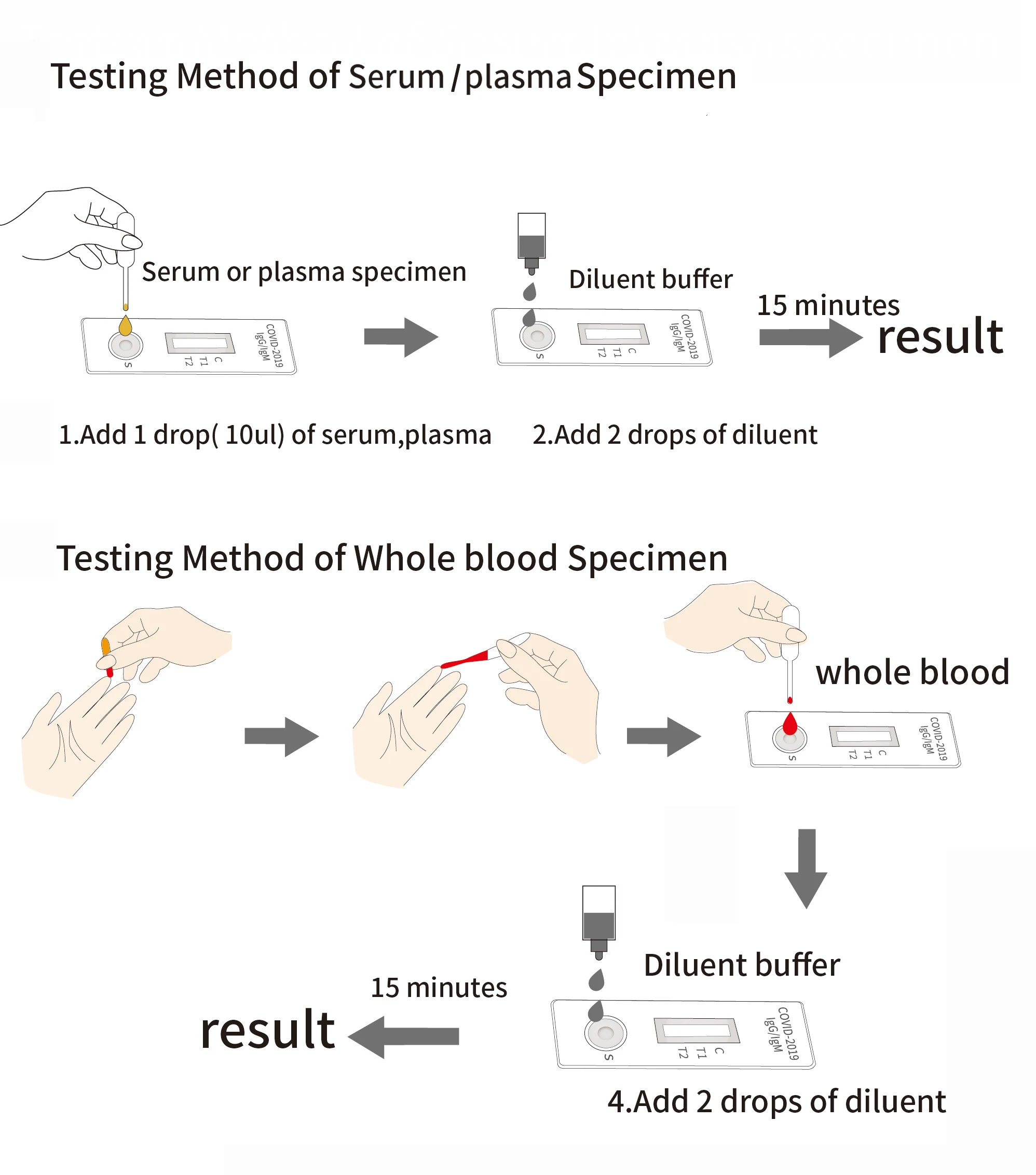
COVID-19 IgG / IgM Ab test is used for qualitative detection of the lgM and IgG antibodies of COVID-19 in human serum / plasma or whole blood.
This kit (colloidal gold method) is a rapid in vitro diagnostic reagent developed by many scientific research institutions with colloidal gold as indicator, colloidal gold immunochromatography technology, anti human IgG and anti human IgM as coating materials, artificial expression and purification of 2019-nCoV specific antigen as labeling materials.








 Luthan Sales
Luthan Sales